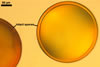
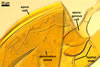
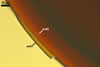
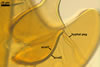
GERMINATION SHIELD
cardioid; hyaline to pale yellow (4A3); 140-185 x 190-200 µm, of a more or less incised border; smooth or ornamented with widely, usually unequally dispersed warts, <0.5 µm high; positioned on the upper surface of the germinal wall. One to 10 holes, from which germ tubes emerge, are present in the wall of this shield.
|
|
|
|
|
|
|
|
In PVLG |
In PVLG+Melzer's reagent |
GERMINATION.
Germ tubes develop from the germination shield and penetrate the spore wall.
AUXILIARY
CELLS borne in the soil, singly or in clusters; pale yellow to pale yellow brown; with blunt, knobby projections (Walker et al. 1993).
MYCORRHIZAE.
According to Walker et al. (1993), S. castanea formed mycorrhizae with arbuscules, coils, and H-connections.
PHYLOGENETIC POSITION. Phylogenetic analyses of Schwarzott et al. (2001) placed S. castanea in the family Gigasporaceae (Diversisporales). According to De Souza et al. (2005), this fungus clustered in the clade C of the Gigasporaceae and its closest relative was S. fulgida.
DISTRIBUTION. Spores of S. castanea have originally been isolated from among roots of Lathyrus sylvestris L. growing on a roadside verge near Pau, Tolouse, France, in 1983 (Walker et al. 1993). Oehl et al. (2005) found this fungus in two permanent grasslands and two mono-cropped maize fields located in the Upper Rhine valley between Basel (Switzerland), Freiburg i. Br. (Germany), and Mulhouse (France).
NOTES. The properties of S. castanea presented above come from the original description of this species (Walker et al. 1993) and examination of its spores obtained from Dr. F. Oehl, Institute of Botany, University of Basel, Switzerland;
fourteen slides labelled BEG01, 2.II.05.
Compared with the properties of spores of S. castanea originally described by Walker et al. (1993), only those of the spore wall of specimens examined by the author of this book slightly differed. Both spore wall layers 1 and 2 were thinner [(0.7-)2.1(-3.2) µm and (11.3-)14.6(19.1) µm thick, respectively] than those given in the protologue [2-4 µm and 10-25(-35) µm thick, respectively].
When observed under a dissecting microscope, three groups of species forming gigasporioid spores may more or less resemble those of S. castanea.
The first group comprises S. arenicola, S. erythropa, and S. hawaiiensis, whose spores are similar in both colour and size to those of S. castanea (Błaszkowski 1992, 2003; Koske and Gemma 1995; Koske and Halvorson 1989; Morton 2002). The second group is represented only by S. armeniaca. Its spores resemble in colour those of the fungus discussed here, but they are markedly smaller [(140-)196(-240) µm diam vs. 169-369 x 176-372 µm in S. castanea; Błaszkowski 1992, 2003; Walker et al. 1993]. Gigaspora margarita and S. aurigloba are members of the third group because of similarity of their darker-coloured spores [sunflower yellow (4A7) and yellow, respectively; Błaszkowski 2003; Walker and Hall 1991] to the lighter-pigmented, maize yellow (4A6), specimens of S. castanea. Additionally, a great size range of spores of Gi. margarita overlaps with that of S. castanea spores [(260-)357(-405) µm diam vs. 169-369 x 176-372 µm, respectively; Błaszkowski 2003; Walker et al. 1993] and the largest spores of S. castanea (369 x 372 µm; Walker et al. 1993) exceed the lower size range of spores of S. aurigloba (323 µm; Walker and Hall 1991).
The most important differences between the species listed above reside in the subcellular structure of their spores, as well as in the phenotypic and biochemical properties of the components of this structure.
While the subcellular structure of spores of S. castanea consists of a spore wall and only one inner germinal wall, that of S. armeniaca, S. aurigloba, and S. hawaiiensis comprises a spore wall and two inner germinal walls (Błaszkowski 1992, 2003; Walker and Hall 1991; Koske and Gemma 1995), and that of S. erythropa a spore wall and three inner germinal walls (Morton 2002). Except for S. armeniaca having a 3-layered spore wall (Błaszkowski 1992; 2003), that of all the other Scutellospora spp. listed above consists of only two layers (Koske and Gemma 1995; Koske and Halvorson 1989; Walker et al. 1993; Walker and Hall 1991). Moreover, the inner layer of the germinal wall of S. castanea spores neither thickens in PVLG nor stains in Melzer's reagent, whereas that of the innermost germinal wall of spores of S. armeniaca and S. hawaiiensis is plastic, increases its thickness several times in PVLG, and stains purple in Melzer's reagent (Błaszkowski 1992, 2003; Koske and Gemma 1995). The inner layer of the second germinal wall of S. aurigloba probably also has such properties (Walker and Hall 1991). To confirm this supposition, tests with living spores crushed in lactic acid-based mountants, such as PVLG, are needed, as Walker and Hall (1991) concluded. Additionally, the innermost layer in the single, 3-layered germinal wall of spores of S. arenicola is plastic and intensively reacts in Melzer's reagent (Koske and Halvorson 1989). However, as results from the protologue, this fungus was described from field-collected spores, in which the spatial distribution of components of their subcellular structure frequently is highly modified (Morton 1993). Spores of S. arenicola probably possess two inner germinal walls, of which the second one comprises a flexible, coriaceous sensu Walker (1986) outer layer and a plastic inner layer, as in most other species of the genus Scutellospora with more than one germinal wall (Błaszkowski 2003; Morton 2002). Ontogenetic studies of spores of S. arenicola are needed to confirm this assumption.
In contrast to all the species of Scutellospora compared above, spores of Gi. margarita do not differentiate any inner germinal wall. Germ tubes of spores of Gigaspora spp. originate from a germinal wall being an integral part of their spore wall (Bentivenga and Morton 1995; Błaszkowski 2003; Morton 2002). Additionally, species of the genera Gigaspora and Scutellospora highly differ in morphology of their auxiliary cells. They are spiny in Gigaspora, and smooth to knobby in Scutellospora (Błaszkowski 2003; Morton 2002).
Apart from S. castanea, the only other representative of the genus Scutellospora producing smooth spores of an identical subcellular structure is S. fulgida, a fungus found to be the closest molecular relative of S. castanea (De Souza et al. 2005). These fungi are easy to separate, because spores of S. fulgida are much lighter [yellowish white (4A2) to cream (4A3) vs. maize yellow (4A6) to brownish orange (6B8); Błaszkowski, pers. observ., 2003] and slightly smaller [(165-)229(-280) µm diam vs. 169-369 x 176-372 µm; Błaszkowski 2003; Walker et al. 1993].
REFERENCES
Bentivenga S. P., Morton J. B. 1995. A monograph of the genus Gigaspora, incorporating developmental patterns of morphological characters. Mycologia 87, 719-731.
Błaszkowski J. 1992. Scutellospora armeniaca, a new species in Glomales (Zygomycetes) from Poland. Mycologia 84, 939-944.
Błaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone , and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. http://www.agro.ar.szczecin.pl/~jblaszkowski/.
De Souza F. A., Declerck S., Smit E., Kowalchuk G. A. 2005. Morphological, ontogenetic and molecular characterization of Scutellospora reticulata (Glomeromycota). Mycol. Res. 109, 697-706.
Koske R. E., Gemma J. N. 1995. Scutellospora hawaiiensis: a new species of arbuscular mycorrhizal fungus from Hawaii. Mycologia 87, 678-683.
Koske R. E., Halvorson W. L. 1989. Scutellospora arenicola and Glomus trimurales: two new species in the Endogonaceae. Mycologia 81, 927-933.
Morton J. B. 1993. Problems and solutions for the integration of glomalean taxonomy, systematic biology, and the study of endomycorrhizal phenomena. Mycorrhiza 2, 97-109.
Morton J. B. 2002. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. West Virginia University. http://www.invam.caf.wvu.edu/.
Oehl F., Sieverding E., Ineichen K., Ris E.-A., Boller T., Wiemken A. 2005. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol. 165, 273-283.
Schwarzott D., Walker C., Schüssler A. 2001. Glomus, the largest genus of the arbuscular mycorrhizal fungi (Glomales) is nonmonophyletic. Mol. Phyl. Evol. 21, 190-197.
Walker C. 1986. Taxonomic concepts in the Endogonaceae. II. A fifth morphological wall type in endogonaceous spores. Mycotaxon 25, 95-99.
Walker C., Gianinazzi-Pearson V., Marion-Espinasse H. 1993. Scutellospora castanea, a newly described arbuscular mycorrhizal fungus. Cryptog. Mycol. 14, 279-286.
Walker C., Hall I. R. 1991. Lectotypification of Scutellospora aurigloba (Glomales). Mycol. Res. 95, 398-400.